Background: Treatment-free remission (TFR) is a new goal for chronic myeloid leukemia (CML) patients who achieved a long deep molecular response (DMR) with better quality of life, reduced adverse events and high economic impact. The primary objective of the AST trial was to estimate molecular relapse rate at 24 month in CP-CML patients who discontinued TKI. Secondary objective to explore predictive markers for sustained TFR through clinical and immunological analysis ( innate inmune response). This update provides longer follow-up data and includes a new cohort of patients.
Patients and Methods: AST-Argentina Stop Trial (NCT05926128) is a multicenter prospective study that included patients from 15 centers. Recruited patients ≥ 18 years, treated with TKI for at least 4 years, sustained MR 4.0 for ≥ 2 years and confirmed typical BCR-ABL1 b3a2 and/or b2a2 transcripts. Molecular studies were centralized in 2 internationally standardized laboratories and were performed monthly during the first 6 months, every 2 months until the first year, and every 3 months during the second year. TKI was restarted due to loss of major molecular response (MMR). Expression of multiple NK cell immunophenotypic markers(CD3-CD56+) was analyzed by flow cytometry at discontinuation time and 3 months later. The nonparametric Mann-Whitney test was used to compare the differences between relapsed and responding patients. Molecular relapse-free survival was estimated using the Kaplan-Meier method and optimal cut-off points were obtained using ROC curves. Comparisons between survival curves were made using the Log rank test method. The differences were considered statistically significant with p-values less than 0.05. Multivariate analysis was performed using the COX regression model.
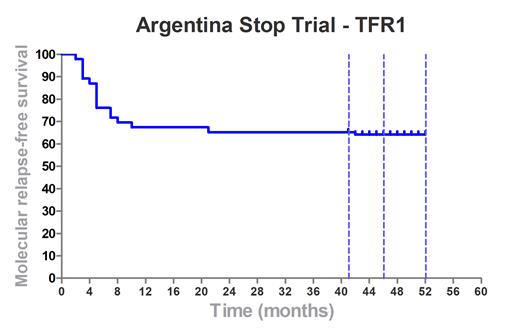
Results: A total of 81 patients divided into 2 cohorts were evaluated: 46 patients recruited between February 2018 and July 2020 (1st cohort) and 35 patients recruited between July 2022 and April 2023. Between these two periods, recruitment was stopped due to the COVID19 pandemic. This analysis focuses on the 1st cohort that has completed 24 months in the study. The median age was 57.5 years (24-86), median duration of treatment before discontinuation was 10.5 years (4.2-20). TKI treatment prior to discontinuation was as follows: Imatinib in 34 (73%) patients, Dasatinib 8 (17%), and Nilotinib 4 (9%). According to Sokal risk score: 22 (48%) were low risk, 14 (30%) intermediate, and 10 (22%) high risk. Seventeen patients (37%) lost MMR, leading to a 63% molecular relapse-free survival rate with a median follow-up of 47 months (41-52). All patients who lost MMR recovered it after restarting the same TKI, with a median time to response of 2.8 months (1-7). With a prolonged follow-up, 2 late relapses were observed (18 and 42 months) (Fig 1). For a univariate analysis, the continuous variables were dichotomized seeking to maximize the difference between the different cohorts. The differences were significant in the variables time in DMR (cut-off point: 51 months, p=0.0166)(HR=0.97 (0.94 - 0.99); p=0.013). Two of the NK cell markers showed significant differences between groups at month 3 after starting treatment, PD1 (Mann-Whitney p=0.035; Log-Rank test p=0.033) and NK-p44 (Mann-Whitney p=0.015; Log -Rank test p=0.020). Multivariate analysis using the Cox model that included variables as gender, age at diagnosis, time in DMR, time in TKI prior to discontinuation, Sokal score, and type of inhibitor, the clinical predictor of time in DMR was the only predictor, significantly associated with a higher rate of TFR, (HR = 0.97 (0.95 - 0.99); p=0.020). No patient experienced disease progression to advanced stages or death for any reason.
Conclusion:This is the first multicenter study of TKI discontinuation in CML patients in Argentina showing that TKI can be safely discontinued in those who achieve and maintain a DMR before discontinuation. Molecular relapse-free survival rate was slightly higher than that reported in the international literature, and longer follow-up confirmed the sustained response. DMR before discontinuation showed to be a robust predictor of TFR. Although the sample size was increased, longer follow-up is needed to validate these findings in the second cohort. Potential advantages of TFR will not only impact in patients' quality of life but also show considerable reduction in the use of economic resources.
Disclosures
Moiraghi:Novartis: Speakers Bureau; Pfizer: Speakers Bureau; Takeda: Speakers Bureau. Pavlovsky:Astra Zeneca: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau. Fernandez:Pfizer: Honoraria; Bristol Myers Squibb, ARG: Honoraria; Astellas Pharma Latin America: Honoraria; Novartis, ARG: Honoraria. Pavlovsky:Bristol Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal